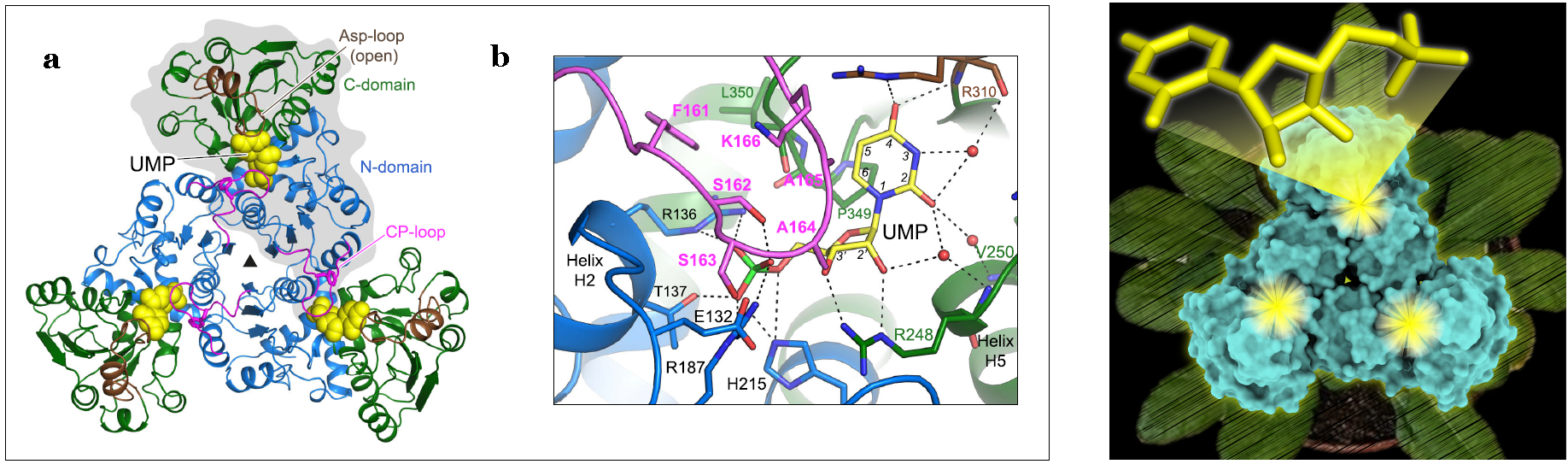
Left: structure of Arabidopsis ATC bound to UMP. a) Crystal structure of atATC trimer with each subunit bound to one molecule of UMP (shown as yellow spheres). One subunit is shown on gray background. b) Detail of the active site with UMP bound. Water molecules are shown as red spheres. Electrostatic interactions are indicated as dashed lines. Right: structure of Arabidopsis ATC in surface representation (in cyan) with the three active sites indicated with a yellow flare. On top, it shows the atomic model of UMP, the natural inhibitor of the protein. The background shows an Arabidopsis plant.
Cerdanyola del Vallès, 7 July 2021 A new work about pyrimidines nucleotides and how they are synthesized in nature has been recently published in Nature Communications. Pyrimidines are organic molecules that are essential compounds of our genetic material and play other prominent roles in many other cellular processes.
In particular, the research team has studied a protein called aspartate transcarbamoylase or ATC, and enzyme with a central role for the synthesis of pyrimidines in all living organisms. Previously, they characterized the structure and function of human ATC. However, not much was known about ATC in plants, other than it has a unique mechanism of regulation, being inhibited by uridine 5-monophosphate (UMP), a product of the metabolic pathway from which all other pyrimidine nucleotides are made.
Using synchrotron light techniques, scientists have determined the first structure of an ATC from plants, that of the plant model Arabidopsis thaliana, free and bound to UMP (the natural inhibitor); and in complex with PALA, a rational-designed inhibitor that mimics the transition-state of the reaction. The structural data obtained at both synchrotrons ALBA and ESRF, combined with mutagenesis and functional assays, allowed the team to describe the nuts-and-bolts of the functioning mechanism of the enzyme and how its activity is regulated in plants.
Scientists made two ground-breaking discoveries: firstly, that unexpectedly, UMP inhibits the enzyme by directly binding and blocking the active site. Secondly, that despite being a trimer with three active sites, the enzyme catalyzes the reaction only in one active site at a time.
From the protein structure to the design of new inhibitors and to the diagnosis of a rare epilepsy
This is a project of basic research, focused on understanding ATC, an ubiquitous enzyme needed for cell growth and proliferation. ATC and other enzymes involved in the synthesis of pyrimidine nucleotides have long been attractive targets for the design of antiproliferative compounds to fight cancer and other diseases. The pharmaceutical Bayer funded part of the project through the Grants4Targets program, for the interest of making ATC inhibitors as potential herbicides. In parallel, the group of Santiago Ramón-Maiques studies ATC in humans, where the enzyme forms part of a much larger protein complex named CAD, an anti-tumoral target that has kept his laboratory busy for the past 10 years. “To selectively inhibit CAD, first, we need to understand how it functions” explains Santiago. Asked about why to study plants when the goal is to manipulate a human protein, he explains “making anti-tumoral compounds against CAD’s ATC activity is difficult. Why not looking first at the solutions found by Nature through millions of years of evolution?” That is what they discovered, the unique solutions found by plants to selectively inhibit ATC. “We learnt an important lesson from our green relatives. The question is how to translate the binding mode of UMP to plant ATC to design new specific anti-proliferative inhibitors against human ATC?” says Santiago when asked about his future research plans.
The group is part of the CIBER of Rare Diseases (CIBERER) and is deeply involved in helping in the correct diagnosis of a rare neurometabolic disease caused by defects in CAD. The disease was first described in 2015, in a 4-year old child with mutations in ATC. “We did not know why the mutation was pathogenic because we did not know how ATC works. The more we learn about the protein, the better we can do in diagnosing children affected by this disease”, explains Santiago. In this regard, the new information on plant ATC, helps to better understand the functioning mechanisms of the human enzyme and to predict the pathogenic potential of clinical variants.
The project is a joined collaboration of the groups of Torsten Möhlmann, Universität Kaiserslautern, and Santiago Ramón-Maiques, Biomedicine Institute of Valencia (IBV-CSIC) and CIBERER. All the structural work was done in the group of Ramón-Maiques. The team counted also with the expertise of Adrián Velázquez-Campoy (Universidad de Zaragoza and ISS Aragón) for doing ITC experiments. The structures determined at the XALOC beamline of ALBA have been key to demonstrate the mechanisms of inhibition and of sequential firing of active sites in plant ATCs.
Reference: Leo Bellin, Francisco Del Caño-Ochoa, Adrián Velázquez-Campoy, Torsten Möhlmann & Santiago Ramón-Maiques. Mechanisms of feedback inhibition and sequential firing of active sites in plant aspartate transcarbamoylase. Nat Commun 12, 947 (2021). https://doi.org/10.1038/s41467-021-21165-9
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the of the Unidades de Cultura Científica y de la Innovación (UCC+i) of the FECYT and has received support through the FCT-20-15798 project.





