Do you want to keep up to date? Subscribe to our newsletter. 1 mail every 2 months! |
 |
Cerdanyola del Vallès, 29th January 2024 The exponential increase in the demand and consumption of fossil fuels has led to rising levels of CO2 concentration in the atmosphere up to reach 420 ppm (part per million) in 2022, which in turn results in global warming and environmental pollution. Different technological alternatives are developed to enable a feasible and gradual transition from carbon-based to renewable fuels. Over the past few years, CO2 capture and utilization (CCU) technology has been regarded as one of the most promising strategies since it can be considered carbon-neutral, contributes to mitigate the fluctuations of renewable energies and significantly reduces the processing costs with respect to CO2 capture and storage (CCS). One of the most viable and sustainable CCU alternative is the CO2 valorisation in the form of methane (CH4), since this reaction does not require additional heat input and the synthetic natural gas (SNG) produced can be stored and transported through existing natural gas infrastructure.
Methanation takes place through adding hydrogen to CO2, producing, as a result, CH4 and water (CO2 + 4H2 ↔ CH4 + 2H2O). The pursuit of active and selective catalysts is essential to accelerate the reaction kinetics advancing towards industrial applications.
Several perovskite-derived formulations have been developed as precursors of efficient catalyst for the CO2 methanation reaction. However, only preliminary mechanistic studies have been carried out on these novel materials.
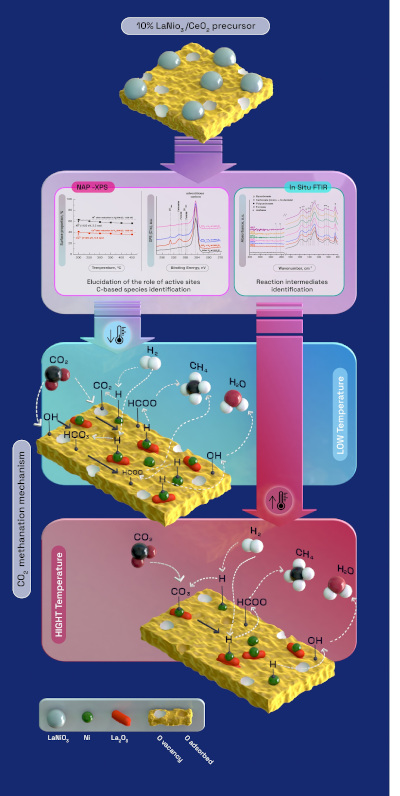 Now, scientists from the University of the Basque Country (UPV/EHU), the University of Alicante and the NAPP branch of the CIRCE beamline of the ALBA Synchrotron have reported a complete scheme of the CO2 methanation mechanism over the highly active, selective and stable 10% LaNiO3/CeO2-derived catalyst; combining for the first time two techniques: near ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) using synchrotron radiation at NAPP-CIRCE beamline and in situ Fourier Transform Infrared Spectroscopy (FTIR) analysis. Researchers show at the paper published in Applied Catalysis B: Environmental that this catalyst performs a high CO2 to methane conversion and stability.
Now, scientists from the University of the Basque Country (UPV/EHU), the University of Alicante and the NAPP branch of the CIRCE beamline of the ALBA Synchrotron have reported a complete scheme of the CO2 methanation mechanism over the highly active, selective and stable 10% LaNiO3/CeO2-derived catalyst; combining for the first time two techniques: near ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) using synchrotron radiation at NAPP-CIRCE beamline and in situ Fourier Transform Infrared Spectroscopy (FTIR) analysis. Researchers show at the paper published in Applied Catalysis B: Environmental that this catalyst performs a high CO2 to methane conversion and stability.
The role and the nature of different catalysts active sites were explored by NAP-XPS experiments. The NAPP branch of CIRCE beamline enabled scientists to monitor the methanation reaction, determining the gas phase composition during the whole experiment, and to control the pressure and the temperature in the analysis chamber.
Catalyst samples and the methanation mixture of CO2 and H2 fed to the analysis chamber were treated under different cooling and heating processes, depending on the experimental protocol defined for the researchers. Different spectra were recorded at each temperature in intervals of 50 °C.
The experiments carried out at CIRCE confirm Ni0 nanoparticles as well as Ni2+–CeO2−x/Ni2+–La2O3 are conformed after reduction. On the other hand, key reaction intermediates are identified by in situ FTIR analysis performed at the TQSA laboratories at the UPV/EHU. Formate acts as main reaction intermediate, which are rapidly hydrogenated to methane.
The insights into the reaction mechanism obtained as a result of this research may be of great help to develop a kinetic model that could accurately predict the catalytic behavior of this novel catalyst for CO2 methanation under industrially relevant conditions and for designing novel formulations with enhanced activity, selectivity and stability in the CO2 methanation reaction. Ultimately, this information is essential for scaling up the process.
Reference: Jon A. Onrubia-Calvo, Sergio López-Rodríguez, Ignacio J. Villar-García, Virginia Pérez-Dieste, Agustín Bueno-López, Juan R. González-Velasco. Molecular elucidation of CO2 methanation over a highly active, selective and stable LaNiO3/CeO2-derived catalyst by in situ FTIR and NAP-XPS. Applied Catalysis B: Environmental. Volume 342, 2024, 123367. ISSN 0926-3373. https://doi.org/10.1016/j.apcatb.2023.123367.




