
Figure. Scanning Electron Microscopy (SEM) data showing changes in the morphology of the material depending on the synthesis temperature.
Cerdanyola del Vallès, 8 April 2021. LNCMO materials, Nickel (Ni) rich layered Li(NixCoyMn1−x−y) O2 oxides, are among the most promising cathode materials for next-generation lithium-ion batteries. These types of batteries are very popular in many of the electronic devices we use every day.
When designing and preparing cathode materials through high-temperature lithiation reaction (adding lithium atoms to the material) it is of fundamental significance to deciphering the sophisticated interplay between thermodynamics and kinetics of the reaction. Whereas thermodynamics is about where the reaction goes, kinetics is about which way it takes to reach the final state.
Researchers already developed scalable methods for the synthesis of these cathodes in a past collaboration with the ALBA Synchrotron. Now, in a study published in Advanced Functional Materials, they show the synthesis optimization of different cathode material for Li-ion batteries, NCM622 (60% nickel, 20% cobalt, and 20% manganese), achieving both lower energy capacity cost and high cyclability. The energy capacity cost is defined as the cost of 1kWh energy storage capacity, and the cyclability is the number of charge/discharge cycles the battery can stand without degradation.
The results can be directly applied to the production of better Li-ion batteries for modern electronics.
The work was carried out by an international research team with collaborators from the Guilin University of Technology in China, the Karlsruhe Institute of Technology (KIT) and the Deutsches Elektronen-Synchrotron (DESY) in Germany and the ALBA Synchrotron in Spain.
Researchers performed a detailed study of the chemical reactions that happen during the formation of NCM622. It appears that by fine-tuning the reaction conditions it is possible to separate stages of:
- initial formation of the layered phase,
- cation (an ion with a positive electric charge) ordering inside it
- and growth of the microscopic crystals, known as crystallites.
By interrupting the reaction immediately after complete cation ordering, it is possible to obtain the material having both the highest possible charging capacity and extremely high capacity retention (ability to retain stored energy) after prolonged cycling.
The reaction was investigated using time-resolved in situ high-resolution X-Ray Diffraction (XRD) technique at the MSPD beamline in the ALBA Synchrotron. “XRD using synchrotron X-rays, is a powerful approach to trace the structural evolution and phase transformation during inorganic reactions, as a contrast to XRD done using laboratory diffractometers which is too slow to follow chemical reactions in situ” explains Alexander Missyul, MSPD beamline scientist.
For a better understanding of the reaction, two complementary approaches were used: heating of the mixture of initial reactants at a constant heating rate up to selected temperature and fast loading of the reaction mixture in the hot zone and following chemical transformations at a constant temperature.
Series of cathode materials obtained at different conditions were investigated electrochemically to find the sample showing the best performance. Additional information was obtained by operando (real working conditions) XRD analysis of the test batteries during first charge-discharge cycles.
The research was supported by X-Ray Absorption Spectroscopy (XAS) data, collected at DESY synchrotron.
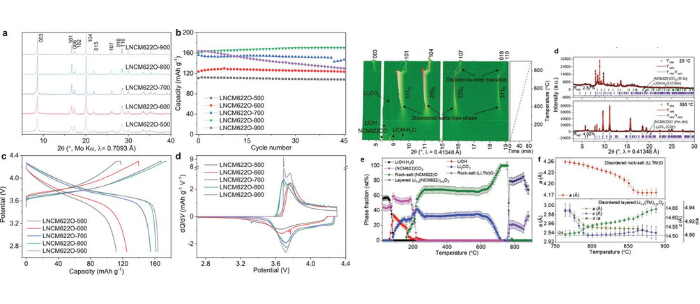
Figure. (a,d)Electrochemical performance of the batteries based on NCM622 obtained at different conditions. (e,f) In situ diffraction data obtained during the synthesis of the NCM622 cathode material.
Reference: Suning Wang, Weibo Hua, Alexander Missyul, Mariyam Susana Dewi Darma, Akhil Tayal, Sylvio Indris, Helmut Ehrenberg, Laijun Liu, and Michael Knapp. Kinetic Control of Long-Range Cationic Ordering in the Synthesis of Layered Ni-Rich Oxides. Adv. Funct. Mater. 2021, 2009949. DOI: 10.1002/adfm.202009949




